Unrivalled performance in terms of safety and respect for the bone material.
The Supercrit® process is the optimal combination of delipidation by supercritical CO2 and a gentle oxidation of the residual proteins.
SUPERCRIT®, TREATMENT OF BONE TISSUE
Various treatment methods have been developed with the aim of devitalizing the bone tissue and making it suitable for the three essential properties expected: validated active viral safety (and no longer by donor selection alone), storage at room temperature in a dehydrated state and, improved osteoconduction by cleaning the bone trabeculae.
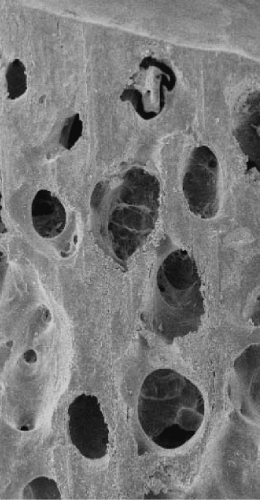
The main challenge is to degrease the cancellous bone tissue which contains many adipocytes in its medullary cavities and potential pathogens. This lipid fraction represents nearly 60% by mass of the fresh cancellous tissue. Its elimination is made all the more difficult by the fact that the trabecular network is dense and thick (Figure 1).
BIOBank is the first tissue bank to have developed and used a high performance, non-toxic fluid, CO2 in a supercritical state. The know-how acquired and developed by BIOBank allows it to master this technology, which offers unequalled performance in terms of safety and respect for the bone matrix.


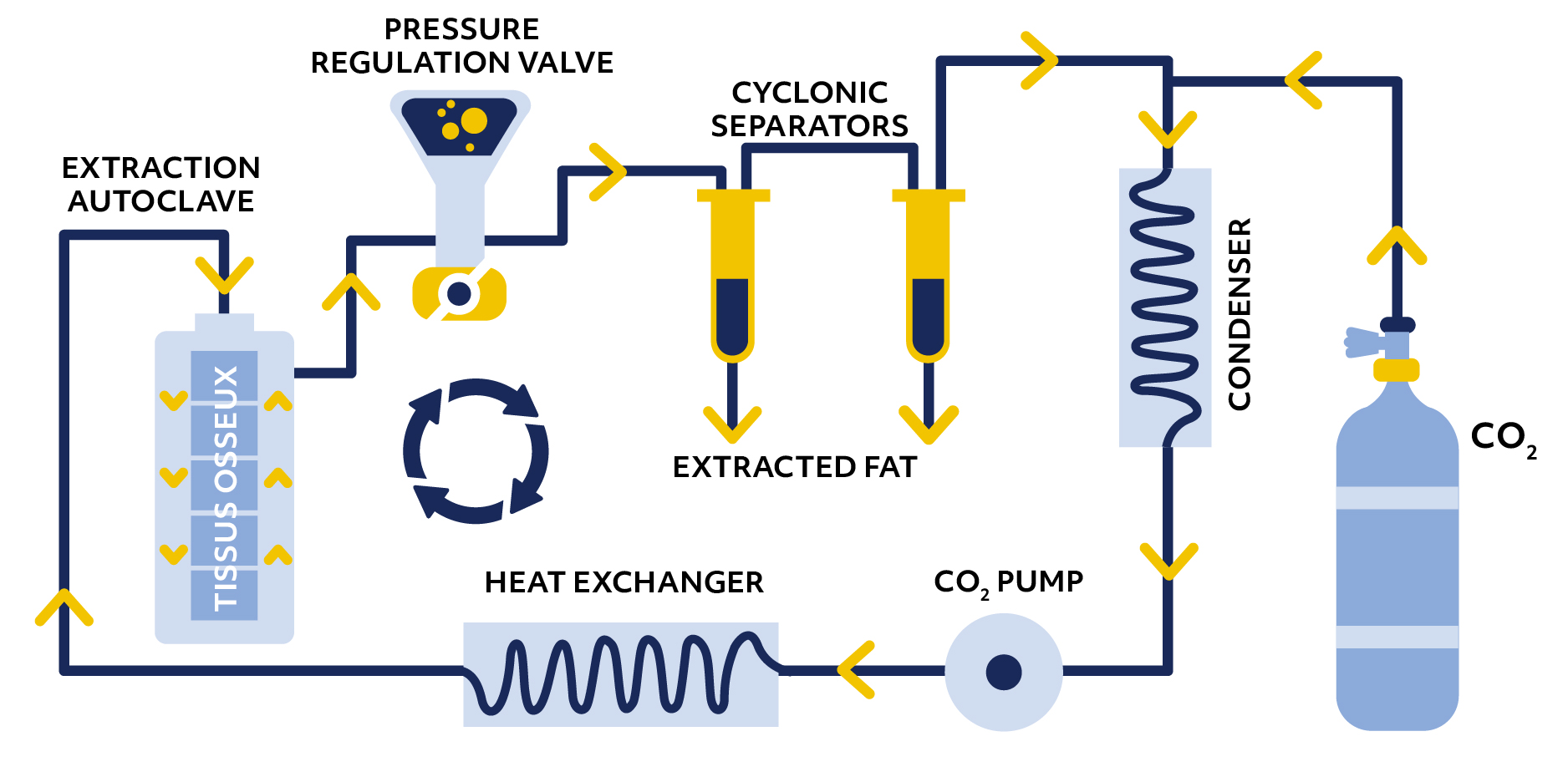
PRINCIPLE AND IMPLEMENTATION OF THE SUPERCRIT® PROCESS
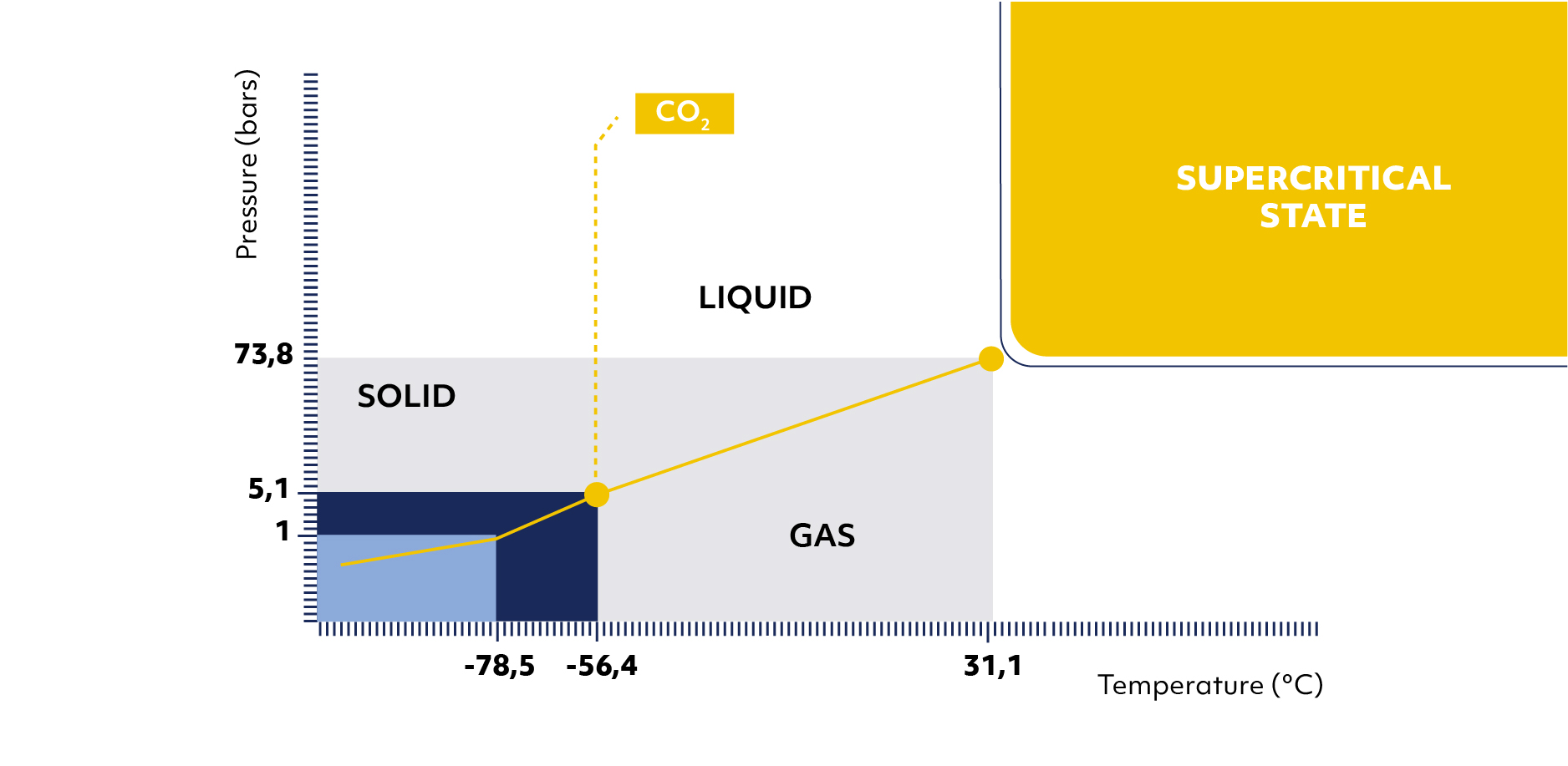
In principle, beyond a critical pressure and temperature, CO2 is in an intermediate state between gas and liquid called supercritical (figure 2) which combines a high solvent power and a very low viscosity (8).
These two properties make this fluid very effective for delipidating bone tissue. Supercritical CO2 is also non-toxic and neutral on the bone structure. It leaves no residue at the end of the treatment. Supercrit® process is a combination of delipidating by supercritical CO2 and chemical oxidation of residual proteins in aqueous solutions.
The preparation of the grafts is carried out in a highly technical environment, combining fully automated equipment operated by highly qualified technicians. Continuous supervision of the environment, quality controls at each stage and microbiological monitoring of the operations guarantee the control of the Supercrit® process.
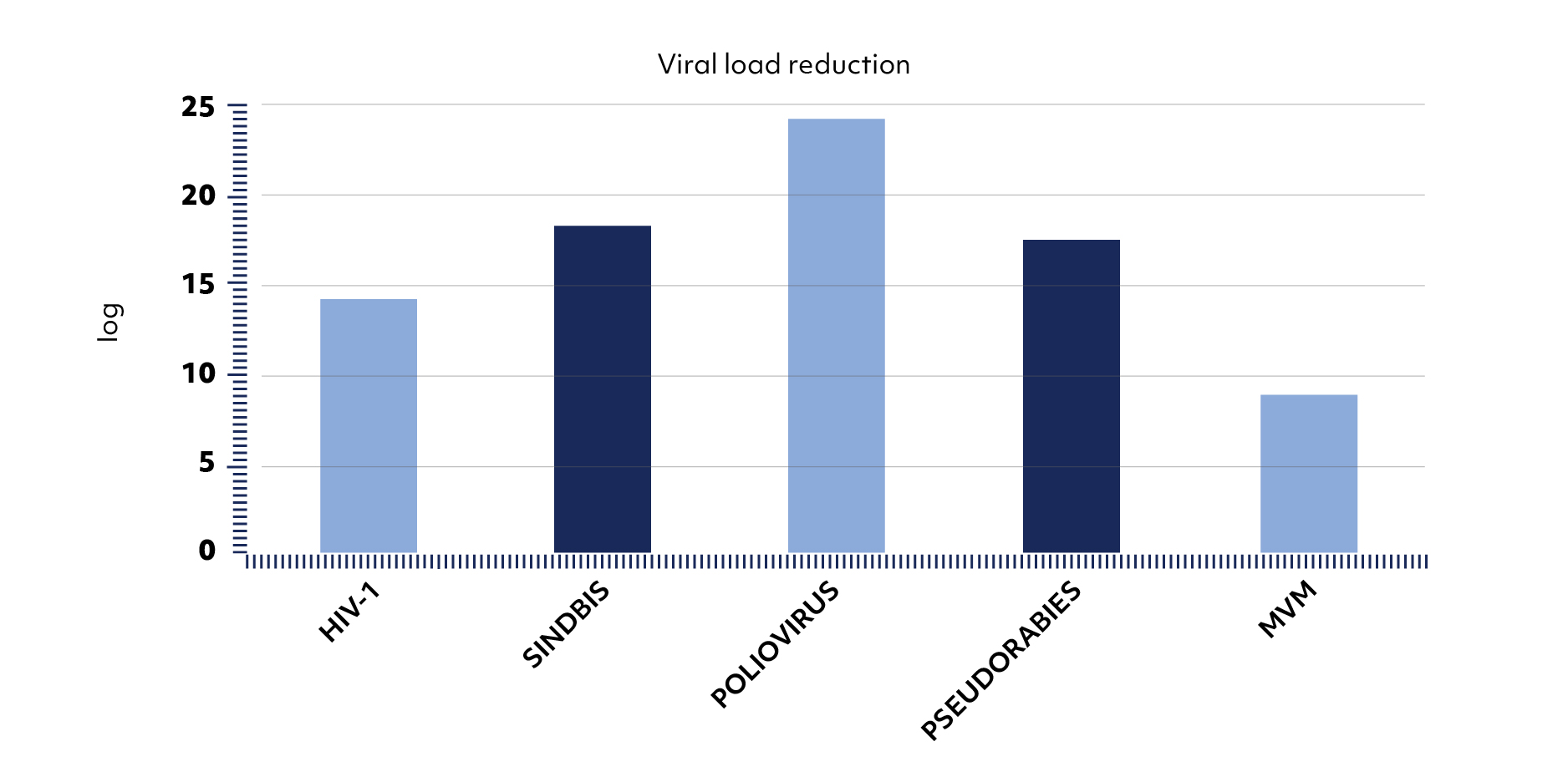
The performances of the Supercrit® process in terms of reduction of a pre-inoculated viral load have been validated (4, 5). The virus inactivating power of the process far exceeds the security assurance level (SAL) of 10-6 required to guarantee the sterility of bone grafts (Figure 3). Evaluation of elasticity and compressive strength of BIOBank bone grafts performed, according to several protocols has demonstrated the specificity of the Supercrit® process to preserve better than others, the architecture and density of the bone tissue (1, 2, 3).
MODE OF USE AND MECHANISM OF ACTION OF BIOBANK BONE GRAFTS
The respect for the integrity of the bone tissue through the Supercrit® process has allowed BIOBank to develop a complete range of products adapted to the specific needs of surgeons. The preservation of grafts in a dehydrated state requires a rehydration phase prior to grafting so that the bone tissue regains its initial elasticity. This can be done with a sterile physiological sodium chloride solution.
Like any inert biomaterials, BIOBank bone allograft acts by osteoconduction. The grafted bone matrix is invaded by vascularisation from the recipient bone bed which provides the bone cells necessary for its remodelling and transformation into a living and functional tissue (6, 7). This mechanism is all the more effective as the Supercrit® process maintains the biological and biomechanical characteristics of the original bone tissue.
What makes the difference with other osteoconductive materials is the intrinsic composition of its mineral-collagenous structure, the preservation of its architecture (Figure 4) and mechanical properties and the particularly strong wettability resulting from the efficiency of the Supercrit® process.

CONCLUSION
All the in vitro and pre-clinical studies validate the pertinence of the Supercrit® process. By combining cleaning and viro-inactivation performance along with the maintaining of the bone structure, it is a modern effective approach. It should be remembered that the implementation of such a process is done within a complex organisation, requiring the mastery of numerous skills while placing rigour at the centre of each stage.
The clinical experience available today, demonstrates the interest in bone allografts and confirms that bone tissue is an exceptional natural biomaterial.
LITERATURE REFERENCES
|
|
01 COMPARATIVE ULTRASOUND EVALUATION OF HUMAN TRABECULAR BONE GRAFT PROPERTIES AFTER TREATMENT WITH DIFFERENT STERILIZATION PROCEDURES.
VASTEL, L., MASSE, C., MESNIL, P., et al.
Journal of Biomedical Materials Research, 2009, Vol.90, N°1, p. 430-437.
|
|
|
02 EFFECTS OF GAMMA IRRADIATION ON MECHANICAL PROPERTIES OF DEFATTED TRABECULAR BONE ALLOGRAFTS ASSESSED BY SPEED OF SOUND MEASUREMENT.
VASTEL, L., MASSE, C., MESNIL, P., et al.
Cell and Tissue Banking, 2007, vol.8, p. 205-210.
|
|
|
03 EFFECT OF A SUPERCRITICAL C02 BASED TREATMENT ON MECHANICAL PROPERTIES OF HUMAN CANCELLOUS BONE.
MITTON, David., RAPPENEAU, Julien., BARDONNET, Raphaël.
European Journal of Orthopaedic Surgery & Traumatology, 2005, N°15, p. 264-269.
|
|
|
04 VIRAL INACTIVATION OF HUMAN BONE TISSUE USING SUPERCRITICAL FLUID EXTRACTION.
FAGES, Jacques., POIRIER, Béatrice., BARBIER, Yves., et al.
ASAIO Journal, 1998, N°44, p. 289-293.
|
|
|
05 BONE ALLOGRAFT AND SUPERCRITICAL PROCESSING : EFFECTS ON OSTEOINTEGRATION AND VIRAL SAFETY.
FAGES, Jacques., JEAN, Eliane., FRAYSSINET, Patrick., et al.
Journal of Supercritical Fluids, 1998, N°13, p. 351-356.
|
|
|
06 HISTOLOGICAL INTEGRATION OF ALLOGENEIC CANCELLOUS BONE TISSUE TREATED BY SUPERCRITICAL CO2 IMPLANTED IN SHEEP BONES.
FRAYSSINET, Patrick., ROUQUET, Nicole., MATHON, Didier., et al.
Biomaterials, 1998, N°19, p. 2247-2253.
|
|
|
07 USE OF SUPERCRITICAL CO2 FOR BONE DELIPIDATION.
FAGES, Jacques., MARTY, Alain., DELGA, Corinne., et al.
Biomaterials, 1994, Vol.15, N°9, p. 650-656.
|


